Background and objectives: CPX-351 is a liposomal formulation of daunorubicin and cytarabine in a fixed synergistic ratio of 5:1. It has been approved by both the FDA and EMA for use in high risk AML including therapy related AML (t-AML) and AML with myelodysplastic related change (AML-MRC). When compared with '3+7', CPX-351 resulted in superior OS (9.56 vs 5.96 months) and overall response rate (47.7% v 33.3%; P = .016; Lancet JCO 2018). However, this is little data of CPX-351 in a real world setting. The primary objective of our multi-centre study was to assess outcomes and associated toxicities of CPX-351 in these high risk AML patients.
Methods: Patients aged 18 and over who were treated with CPX-351 and who met the WHO criteria for t-AML and AML-MRC were included in the study. Retrospective data was collected from 6 centres throughout Canada. Targeted sequencing was performed on DNA samples using the TruSight Myeloid Sequencing Panel isolated from peripheral blood or bone marrow samples at diagnosis. Overall survival (OS) and progression free survival (PFS) rates were calculated using the Kaplan-Meier method.
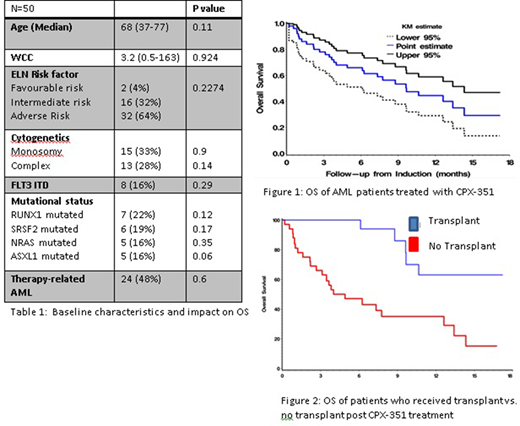
Results: Fifty patients treated with CPX-351 were identified from six centres. Baseline characteristics are seen in Table 1. Cytogenetics was available in 45 (90%) patients and an abnormal karyotype was seen in 30 (60%) patients. Monosomal (33%) and complex (28%) karyotype were the most common chromosomal abnormalities seen. Targeted sequencing was performed on 64% patients (32/50) and the average number of mutations was 2(0-7). RUNX1 was the most commonly mutated gene found in 22% (7/32), followed by SRSF2 in 19% (6/32) with ASXL1 and NRAS both at 16% (5/32). Other commonly mutated genes were NPM1, IDH2, DNMT3a and TP53 all occurred at a frequency of 12% (4/32). Of the 10 patients (20%) that were FLT3 mutated (80% ITD and 20% TKD), 5 patients received the FLT3 inhibitor midostaurin in combination with CPX-351.
Assessing treatment responses, 50% (25/50) of patients achieved a complete remission (CR) or a complete remission with incomplete recovery (CRi). Median neutrophil recovery (≥500/μl) was 32 days (range 18-68) and median platelet recovery (≥50 000/μl) was 34 days (range 19-70). Proven or probable fungal infection was seen in 10 (20%) patients. 30 day mortality was 4% (2/50) and 60-day mortality was 10% (5/50). The median CR duration was short at 7.17 months. Of the 25 patients who did not achieve a CR, 6 (24%) had prior azacitidine use for MDS suggesting CPX-351 may not have activity in this group of patients.
Median follow up was 7.43 months (range 0.2 to 18 months). OS was 44% at 12 months (0.28-0.58 95% CI) and 29% at 18 months (Fig 1; 0.13-0.46 95% CI). PFS was 28% at 12 months (0.15-0.43 95% CI) and 18% at 18 months (0.06-0.39 95% CI). There were no differences in OS when stratified by ELN risk (p=0.25), adverse risk cytogenetics (p=0.1485), or poor risk mutations such as FLT3-ITD (p=0.29), RUNX1 (p=0.123) or ASXL1 (p=0.06). This is consistent with previous studies, which suggest that CPX-351 overcomes the poor prognosis associated with these mutations.
18 (36%) patients received an allogeneic stem cell transplant (ASCT) in CR1. Patients who received a allogeneic transplant had significant improvement of OS of 62% at 18 months compared to those who did not receive a transplant of 14.5% at 18 months (Fig 2; p=0.0008).
Conclusion
Our study reveals that CPX-351 produces high remission rates in patients with AML with a similar efficacy and toxicity profile seen in the Phase 3 trial data (Lancet JCO 2018). However, response rates are short and therefore CPX-351 is most effective when used as a bridge for allogeneic stem cell transplantation in this high risk population.
Assouline:AbbVie: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Takeda: Research Funding; BeiGene: Consultancy, Honoraria, Research Funding. Brandwein:Celgene: Honoraria; Astellas: Honoraria; Taiho: Honoraria; Roche: Honoraria; Jazz Pharmaceuticals: Honoraria; Pfizer: Honoraria; Amgen: Honoraria. Gupta:Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Research Funding; Bristol MyersSquibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Maze:Pfizer: Consultancy; Takeda: Research Funding; Novartis: Honoraria. McNamara:Novartis: Honoraria. Sanford:Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Schimmer:Medivir AB: Research Funding; AbbVie Pharmaceuticals: Other: owns stock ; Takeda: Honoraria, Research Funding; Novartis: Honoraria; Jazz: Honoraria; Otsuka: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal